|
Ark Health |

|
|---|
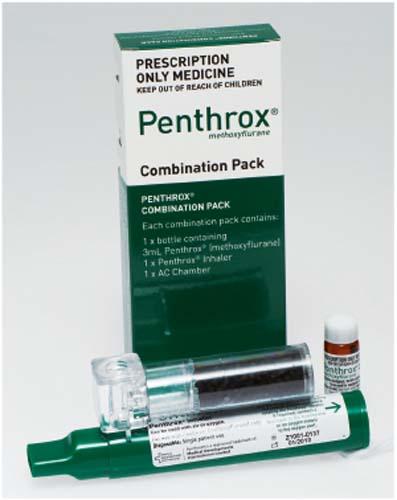
Penthrox Combo Pack with AC Chamber (SCHEDULE 4)
Penthrox is your ideal Pre-injection analgesic
Penthrox can be administered:
- During uncomfortable procedures that may cause patients pain
- Initial examination
- Emergency situations
Kit Contains:
- 1 x bottle containing 3mL Penthrox (methoxyflurane)
- 1 x Penthrox Inhaler
- 1 x AC Chamber
Penthrox is a SCHEUDLE 4 drug in Australia.
To purchase this product you are required to be a registered Health Care Professional with AHPRA. Additional conditions may apply.
For further information contact 1800 PENTHROX (1800 736 847)
MINIMUM PRODUCT INFORMATION:
Penthrox(methoxyflurane) Inhalation Before prescribing, please review Approved Product Information. Full PI available from Medical Developments International Limited.
INDICATIONS:
For emergency relief of pain by self administration in conscious haemodynamically stable patients with trauma and associated pain, under supervision of personnel trained in its use and for the relief of pain in monitored conscious patients who require analgesia for surgical procedures such as the change of dressings.
CONTRAINDICATIONS:
Use as an anaesthetic agent renal impairment renal failure hypersensitivity to fluorinated anaesthetics (including familial history of hypersensitivity) cardiovascular instability respiratory depression head injury or loss of consciousness malignant hyperthermia.
PRECAUTIONS:
Not to be used as an anaesthetic agent liver disease and liver damage after previous methoxyflurane or halothane anaesthesia diabetic patients (may have an increased likelihood of developing nephropathy) daily use of methoxyflurane is not recommended treatment with enzyme inducing drugs (e.g. barbiturates) cautious use of adrenaline or nor-adrenaline during methoxyflurane administration caution in hot climates (do not expose to temperatures above 40°C, especially when used in conjunction with oxygen) use in pregnancy, the elderly and regular exposure to health workers.
INTERACTIONS:
Antibiotics including tetracycline, gentamicin, kanamycin, colistin, polymyxin B, cephaloridine and amphotericin B subsequent narcotics administration -blockers.
SIDE EFFECTS:
With analgesic doses - euphoria, drowsiness, sleepiness, agitation, restlessness, headache, dizziness, dissociation, amnesia, cough, choking, hypotension, nausea, vomiting, hepatitis, increased liver enzymes, increased serum uric acid, urea nitrogen and creatinine, blurred vision, diplopia and nystagmus. Hepatic toxicity in association with methoxyflurane is rare.
DOSAGE & ADMINISTRATION:
Up to 6 mL per day, vaporised in a PENTHROX Inhaler. The total weekly dose should not exceed 15 mL. Administration on consecutive days is not recommended. Date prepared: August 2012.
Product Code: 354221
Safety Data Sheets:
Popular Items
Penthrox Combo Pack with AC Chamber (SCHEDULE 4)Product Code: 354221 Category: Anaesthesia
Price: $59.00 (inc GST) Brand: MDI Weight: 1 Description:
Penthrox is your ideal Pre-injection analgesic Penthrox can be administered:
Kit Contains:
Penthrox is a SCHEUDLE 4 drug in Australia. To purchase this product you are required to be a registered Health Care Professional with AHPRA. Additional conditions may apply. For further information contact 1800 PENTHROX (1800 736 847) MINIMUM PRODUCT INFORMATION: Penthrox(methoxyflurane) Inhalation Before prescribing, please review Approved Product Information. Full PI available from Medical Developments International Limited. INDICATIONS: For emergency relief of pain by self administration in conscious haemodynamically stable patients with trauma and associated pain, under supervision of personnel trained in its use and for the relief of pain in monitored conscious patients who require analgesia for surgical procedures such as the change of dressings. CONTRAINDICATIONS: Use as an anaesthetic agent renal impairment renal failure hypersensitivity to fluorinated anaesthetics (including familial history of hypersensitivity) cardiovascular instability respiratory depression head injury or loss of consciousness malignant hyperthermia. PRECAUTIONS: Not to be used as an anaesthetic agent liver disease and liver damage after previous methoxyflurane or halothane anaesthesia diabetic patients (may have an increased likelihood of developing nephropathy) daily use of methoxyflurane is not recommended treatment with enzyme inducing drugs (e.g. barbiturates) cautious use of adrenaline or nor-adrenaline during methoxyflurane administration caution in hot climates (do not expose to temperatures above 40°C, especially when used in conjunction with oxygen) use in pregnancy, the elderly and regular exposure to health workers. INTERACTIONS: Antibiotics including tetracycline, gentamicin, kanamycin, colistin, polymyxin B, cephaloridine and amphotericin B subsequent narcotics administration -blockers. SIDE EFFECTS: With analgesic doses - euphoria, drowsiness, sleepiness, agitation, restlessness, headache, dizziness, dissociation, amnesia, cough, choking, hypotension, nausea, vomiting, hepatitis, increased liver enzymes, increased serum uric acid, urea nitrogen and creatinine, blurred vision, diplopia and nystagmus. Hepatic toxicity in association with methoxyflurane is rare. DOSAGE & ADMINISTRATION: Up to 6 mL per day, vaporised in a PENTHROX Inhaler. The total weekly dose should not exceed 15 mL. Administration on consecutive days is not recommended. Date prepared: August 2012. |
 |